Abstract
Introduction: In systemic light-chain amyloidosis (AL) aberrant clonal free immunoglobulin light chains (FLC) misfold and deposit in vital organs causing severe dysfunction (Nat Rev Dis Primers 2018;4:38). With anti-plasma cell therapy that reduces or eliminates the involved FLC (iFLC), defined organ responses can occur (N Engl J Med 2021;385:46, Blood Rev 2019;37:100581, Leukemia 2017;31:136, Blood 2014;124:2325). We asked whether the timing of individual organ responses may be influenced by the number of organs involved at diagnosis; therefore we evaluated the pattern of responses in patients with the two most commonly involved organs (heart, kidney) who achieved deep hematologic responses to therapy (CR=complete response, VGPR=very good partial response)(J Clin Oncol 2012;30:4541). We examined whether the rate of and time to organ response varied in patients with only heart or kidney or heart and kidney involvement, and whether the depth of hematologic response impacted the pattern of organ response.
Methods: We performed a retrospective analysis AL patients diagnosed by tissue biopsy between 2007-2019 who had heart and/or kidney involvement at diagnosis and achieved hematologic CR/VGPR with treatment. Mann-Whitney was used to compare rates of organ responses and log-rank tests were applied to compare times to organ response among the subgroups as well as overall survival (OS) differences based on iFLC responses and on organ responses. Results were considered to be significant if two-sided P-value was less than or equal to 0.05.
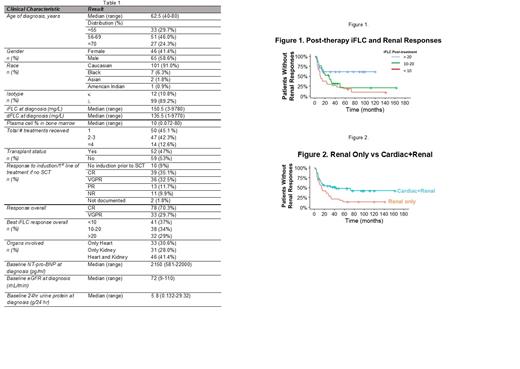
Results: We identified 111 patients with a median age of 62.5 years (range, 40-80) who met these criteria, 65 of whom (59%) were male. Cardiac involvement only was present in 34 (30.6%), renal involvement only in 31 (28.0%), and both cardiac and renal involvement in 46 (41.4%). Table 1 highlights patient characteristics. The median OS for the entire cohort was 112 months (95% CI 100-NA). The overall cardiac response rate was 62.5%, with a median time to response of 8 months (range, 1-73 months). Overall renal response rate was 67.1% with a median time to response of 10 months (range, 1-57 months). Log-rank analysis showed a significant difference in the OS based on post treatment iFLC levels (<10 vs. 10-20 vs. >20 mg/L) as we have previously described (Am J Hematol 2021;96:E20). Patients with kidney involvement only had significantly improved overall survival (OS) compared to those with cardiac involvement only (p=0.05), as expected. However, there was no difference in the OS of patients with cardiac only vs. cardiac and renal involvement (p=0.58), while there was a trend towards shorter OS in patients with cardiac and renal vs renal (p=0.09). The lower iFLC levels achieved post-treatment influenced cardiac response rate (p=0.07), and significantly impacted renal response rate (p<0.01). For patients with cardiac involvement, iFLC responses did not have a significant impact on time to cardiac response, whereas for patients with renal involvement, faster responses were noted in those achieving lower iFLC levels (p=0.017) (Figure 1). There was no significant difference in time to cardiac response between patients with cardiac only vs. cardiac and renal involvement (p=0.93) whereas patients with renal only vs cardiac and renal involvement had a faster time to renal response (medians 14 (range, 10-29) vs 43 (13-not reached) months, p=0.018) (Figure 2).
Conclusion: In AL patients with renal involvement who achieve CR/VGPR with treatment, post-treatment iFLC levels and co-presence of cardiac involvement play significant roles in the timing of renal responses. In AL patients with cardiac involvement who achieve CR/VGPR, post-treatment iFLC levels but not the co-presence of renal involvement influences the rate of cardiac response but neither influences the timing. These differences may be due to organ-specific factors such as proteomic adaptations or relative iFLC toxicity or complex cardio-renal hormonal interactions. Further hypothesis-driven study of these differences is warranted in this era of new and effective anti-plasma cell therapies.
Comenzo: Prothena Biosciences: Consultancy, Research Funding; Karyopharm: Research Funding; Takeda: Research Funding; Unum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Caelum: Consultancy, Research Funding; Janssen: Patents & Royalties: WO2016187546A1, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal